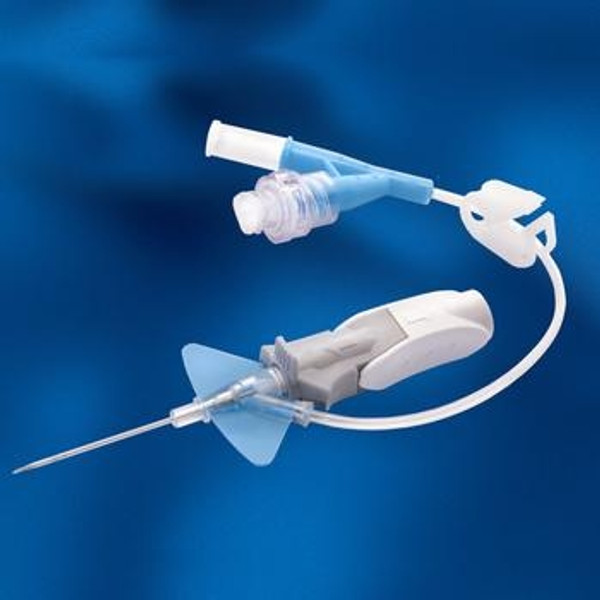
Product Overview
BD Nexiva Closed IV Catheter System made of BD Vialon Biomaterial with Dual Port & Luer Access Devices has been designed to:
- Reduce insertion attempts
- Increase protection against blood exposure and needlestick injuries
- Simplify IV therapy process
- Enhance patient safety and satisfaction
- Sterile. Latex-free.
20/Shelf Pack; 80/Case.
- Reduce insertion attempts
- Increase protection against blood exposure and needlestick injuries
- Simplify IV therapy process
- Enhance patient safety and satisfaction
- Sterile. Latex-free.
20/Shelf Pack; 80/Case.
Part Number(s):
- BND383537BX - 20 ga x 1 1/4" HF Dual Port (1.1 x 31 mm) - 1 box (20 Each)
- BND383537CS - 20 ga x 1 1/4" HF Dual Port (1.1 x 31 mm) - 1 case (80 Each)
- BND383536BX - 20 ga x 1" HF Dual Port (1.1 x 25 mm) - 1 box (20 Each)
- BND383536CS - 20 ga x 1" HF Dual Port (1.1 x 25 mm) - 1 case (80 Each)
- BND383532BX - 22 ga x 1" Dual Port (0.9 x 25 mm) - 1 box (20 Each)
- BND383532CS - 22 ga x 1" Dual Port (0.9 x 25 mm) - 1 case (80 Each)
- BND383531BX - 24 ga x 3/4" Dual Port (0.7 x 19 mm) - 1 box (20 Each)
- BND383531CS - 24 ga x 3/4" Dual Port (0.7 x 19 mm) - 1 case (80 Each)

 Help
Help